Project Summary
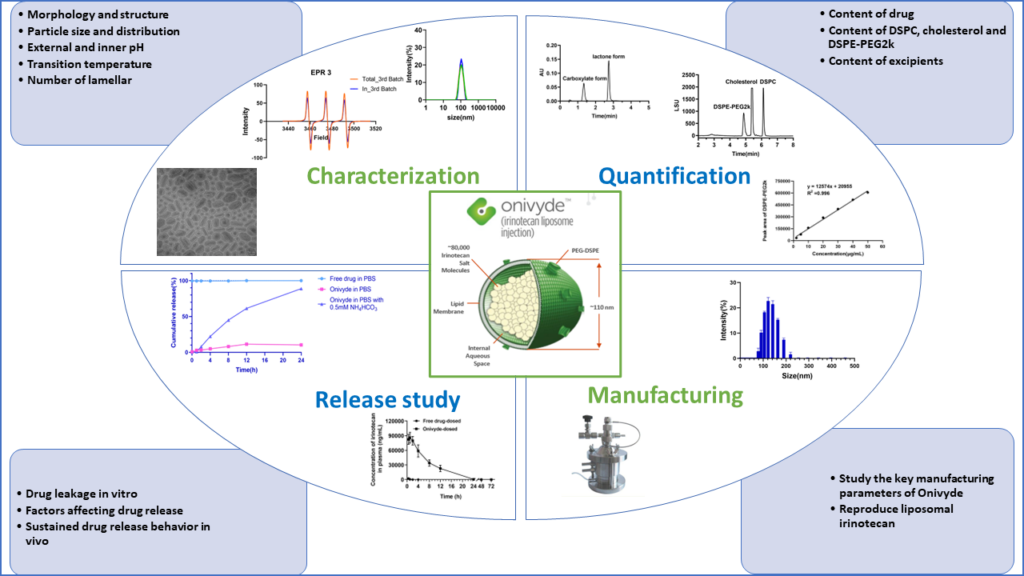
Based on insights from interviews and surveys conducted by CRCG, we identified the need for research focused on “reverse engineering” of complex generic products and in vitro release (IVR) methodologies for liposomal products due to inherent difficulties in validating IVR methodologies and challenges in showing their discriminative abilities between batches of liposomes at various stages of the development cycle.
This project will focus on analytical characterization ONIVYDE™, an irinotecan liposome injection for intravenous use, establishing a USP-4 apparatus based IVR assay to examine the release of irinotecan from the liposomes and small-scale manufacturing of irinotecan liposome injection.